12+ Chapter 7 Ionic And Metallic Bonding
73 Lewis Symbols and. IiMetallic character With the exceptions of Zn Cd and Hg they have typical metallic structures.
Nicotine C10h14n2 Pubchem
When such an electron transfer takes place one of the atoms develops a negative charge and is now called the anion.

. Where X is any atom or molecule X. Ionic bonding is a type of chemical bonding which involves a transfer of electrons from one atom or molecule to another. Here an atom loses an electron which is in turn gained by another atom.
Writing Ionic Compound Formulas. Ionic compounds are non-directional and do not. Our Custom Essay Writing Service Features.
Some noble gases also combine with oxygen and F to form a no. Lesson 7 - Ionic Compounds. Binary Polyatomic Compounds 1242 Covalent Compounds.
Single Double Triple Bonds 509. Properties Naming Formation 1021 Lewis Structures. The main idea behind this chapter is the.
Write the balanced ionic equation for the reaction between ferrous sulphate and acidified potassium permanganate solution. NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download. We will guide you on how to place your essay.
The first ionization energy is quantitatively expressed as Xg energy X g e. Here we are providing Class 11 chemistry Important Extra Questions and Answers Chapter 4 Chemical Bonding and Molecular Structure. Comprehensive student-friendly answers are provided according to the latest CBSE Syllabus 2022-23 to all the in-text and exercise.
Single Double Triple Bonds 509. Section you will be able to. The alkali metals consist of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr.
Writing Ionic Compound Formulas. In chemistry and physics the exchange interaction with an exchange energy and exchange term is a quantum mechanical effect that only occurs between identical particlesDespite sometimes being called an exchange force in an analogy to classical force it is not a true force as it lacks a force carrier. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution.
NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements are powerful study materials that have answers to textbook exercises and important questions from previous years and sample papers. Predict the charge of common metallic and nonmetallic elements and write their. Once dissolved or melted ionic compounds are excellent conductors of electricity and heat because the ions can move about freely.
Properties Naming Formation 1021 Lewis Structures. Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off. This shared electron configuration results in their having very similar characteristic.
The latest Lifestyle Daily Life news tips opinion and advice from The Sydney Morning Herald covering life and relationships beauty fashion health wellbeing. In addition to metallic bonding there is extra covalent bonding due to presence of unpaired electrons in. Bonding between a metal and a nonmetal is often ionic.
Get 247 customer support help when you place a homework help service order with us. Where A is the Hamaker coefficient which is a constant 10 19 10 20 J that depends on the material properties it can be positive or negative in sign depending on the intervening medium and z is the center-to-center distance. IiiAtomic and ionic size-ions of same charge in a given series show progressive decrease in radius with increasing atomic number.
IF 7 the central atoms like P S I have 101214 electrons respectively. Ionic bonding is a sort of chemical bonding that occurs when electrons are transferred from one atom or molecule to another. NCERT Solutions Class 11 Chemistry Chapter 3 Free PDF Download.
Join an activity with your class and find or create your own quizzes and flashcards. Resistivity is commonly represented by the Greek letter ρ The SI unit of electrical resistivity is the ohm-meter Ωm. Explain the formation of cations anions and ionic compounds.
Single Double Triple Bonds 509. Microsoft describes the CMAs concerns as misplaced and says that. Video 18 min U.
Together with hydrogen they constitute group 1 which lies in the s-block of the periodic tableAll alkali metals have their outermost electron in an s-orbital. Ionic solids are also poor conductors of electricity for the same reasonthe strength of ionic bonds prevents ions from moving freely in the solid state. Some compounds contain both covalent and ionic bonds.
Most ionic solids however dissolve readily in water. Air for example is a solution. Writing Ionic Compound Formulas.
Binary Polyatomic Compounds 1242 Covalent Compounds. Ie the sum of R 1 R 2 and r the distance between the surfaces. The van der Waals force between two spheres of constant radii R 1 and R.
NCERT Solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties are provided on this page for CBSE Class 11 students. The effect is due to the wave function of indistinguishable particles. Bonds between two nonmetals are generally covalent.
Chemical Bonding is Chapter 4 of Class 11 Chemistry. Important Questions for Class 12 Chemistry Chapter 8 The d- and f-Block Elements Class 12 Important Questions. The atoms in polyatomic ions such as OH NO 3 NO 3 and NH 4 NH 4 are held together by polar covalent bonds.
Ranging from2 to 7. Of compounds like. Electrical resistivity also called specific electrical resistance or volume resistivity is a fundamental property of a material that measures how strongly it resists electric currentA low resistivity indicates a material that readily allows electric current.
Binary Polyatomic Compounds 1242 Covalent Compounds. Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. Lesson 20 - Metallic Bonding.
One atom loses an electron in this process which is then gained by another atom. To perform well in the Class 12 Chemistry board exam students must understand Chapter 1 thoroughly. Solutions are all around us.
Chapter 12 Practice Test Practice test. Properties Naming Formation 1021 Lewis Structures. Psychiatric interviews for teaching.
In physics and chemistry ionization energy IE American English spelling ionisation energy British English spelling is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom positive ion or molecule. The state of matter forms the fundamentals for many chapters later on. Class 12 Chapter 1 The Solid State is an essential chapter that helps you to understand the basics of Chemistry as it focuses on the State of Matter.
These NCERT Solutions for Class 12 Chemistry are prepared based on the latest.

Chapter 7 Ionic And Metallic Bonding Ppt Download

Ionic Transport Properties Of La X Y 3 X Fe 5 O 12 D Compounds A The Download Scientific Diagram
Pdf 7 Transition Metal Chemistry

Minerals Earth Home

What Are The Main Properties Of Ionic Covalent Bonds And Metallic Bonds Quora

Preview Chemistry For The Ib Diploma Exam Preparation Guide By Cambridge University Press Education Issuu

Ionic And Metallic Bonding Pdf Free Download
Frontiers Coordination Clusters Of 3d Metals That Behave As Single Molecule Magnets Smms Synthetic Routes And Strategies

Pdf Chap 7 Ionic Bonding Asad Bhatti Academia Edu
Chemistry Chapter 7 Ionic And Metallic Bonding Heart Cell Rhythm Depends On The Opening And Closing Of A Complex Series Of Valves On The Cell Membrane Ppt Download
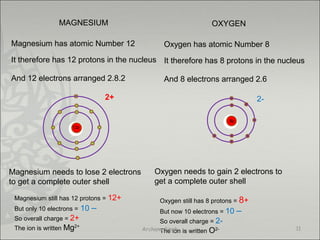
Ionic Bond
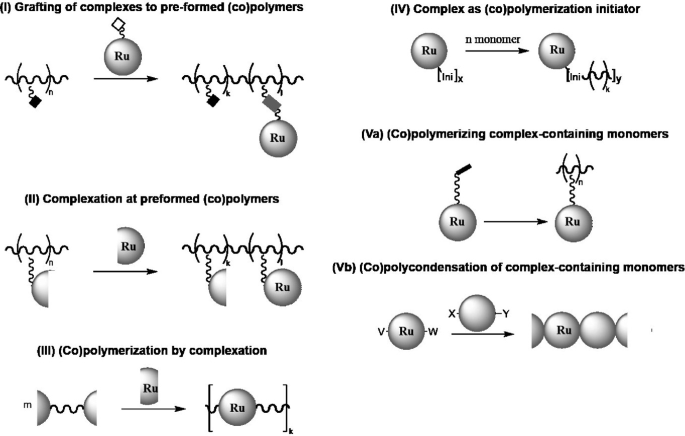
Polymers Incorporating Ru Complexes Springerlink

Ionic Or Electrovalent Compounds Properties Characteristics With Videos

Coordination Compounds Class 12 Notes Chemistry Chapter 9 Samar Education

Ionic And Metallic Bonding Pdf Free Download

Pdf Metallosupramolecular Helicates And Tetrahedra Transition Metal Directed Assembly Of Polypyridyl Ligands Christopher Glasson Academia Edu

Coordination Compounds Class 12 Notes Chemistry Chapter 9 Samar Education